cation vs anion
Frequently Asked Questions FAQs on Cations and Anions. Cations are ions that are positively charged.
 |
| Ions Concept 2 Pearson Channels |
A positive-charged ion or charged particle is referred to as a cation.

. Between anions or cations which one is bigger. But for the anion formation the. Cations vs anions whats the difference. When an atom loses one or more electron in order to gain more stability it becomes the positively charged ion Cation and has the positive sign on it denoting its ability.
An atom that permanently loses electrons becomes a cation ie positively charges ion. An anion is an ion or a charged particle with a negative charge. A cation has a net positive electrical charge which means it has more protons than electrons. It becomes positive called a cation.
In cation the protons number is greater than the number. Anions are larger whereas cations are smaller than their. To form a cation a neutral atom has to donate its electrons. Cl has gained a negative charge an electron and it becomes negative.
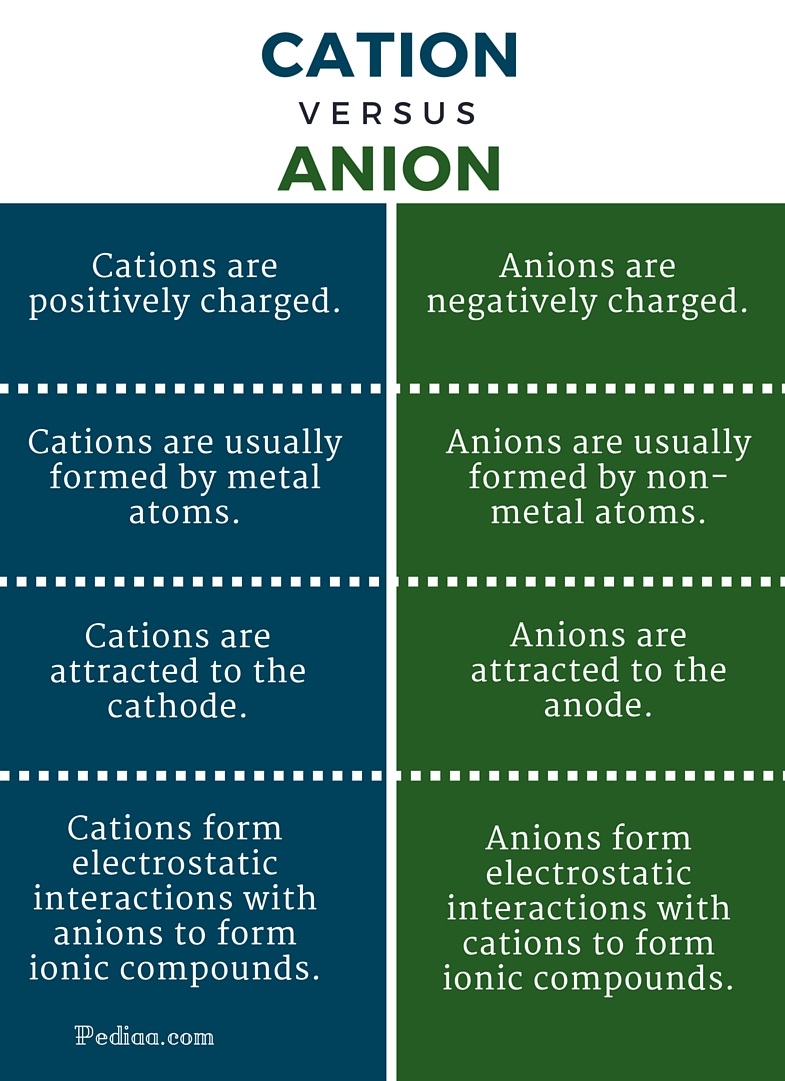
The main difference between Cation and Anion is that Cation has a positive charge whereas anion has a negative charge. Cation is a related term of ion. Cation is a positively charged ion because it has more protons than electrons and anion is a negatively charged ion because it has more electrons than protons. The number of protons in.
An anion has a net negative electrical charge. Anions are ions that are negatively charged. Cation has more protons than electrons. As nouns the difference between cation and ion is that cation is chemistry a positively charged ionopposed to anion while ion is an atom or group of atoms.
The oppositely charged atoms attract and you have an. What are Cations and Anions. The cations are positively charged ions while the anions are negatively charged ions. The first difference is that anions possess negative electrical charges while the cations have positive electrical charges.
There are two types of ions. Ions are particles that do not have a balanced or neutral number of positively-charged protons and negatively-charged electrons. Ions are charged atoms or molecules. Differences Between a Cation and an Anion.
Anion has more electrons than protons.
 |
| How Ions Are Formed Cation Vs Anion Best Chemistry Blog Digital Kemistry Best Online Free Chemistry Learning |
 |
| Theoretical Insight Into Effect Of Cation Anion Pairs On Co2 Reduction On Bismuth Electrocatalysts Sciencedirect |
 |
| Bjoc Halides As Versatile Anions In Asymmetric Anion Binding Organocatalysis |
 |
| Difference Between Cation And Anion |
 |
| What Is An Ion Cation Vs Anion Definition Difference Examples Most Important Chemistry Topic Science Chemistry Chemistry Definitions |
Posting Komentar untuk "cation vs anion"